Organic Chemistry and Introduction For Nurses

Organic compounds
In early 19th century, Swedish
chemist Jacob Berzellius put forward the “Vital Force Theory”.According to this
theory, organic compounds could not be prepared in laboratories because they
were supposed to be synthesized under the influence of a mysterious force
called Vital Force, inherent only in living things.
The Vital Force theory suffered
death blow in 1828 when Wohler synthesized the firstorganic compound urea from
inorganic substance by heating ammonium cyanate (NH4CNO):
Organic chemistry
The branch of chemistry which deals with the study
of hydrocarbons and their derivatives is known as organic chemistry.
There are four types of formulae of organic compounds:
• Molecular formula
• Structural formula
• Condensed formula
• Dot and cross formula
Molecular Formula
The formula which represents the actual number of atoms in
one molecule of the organic compound is called the molecular formula, e.g.,
molecular formula of butane is C4H10. It shows:
a. Butane is made up of carbon and hydrogen atoms.
b. Each molecule of butane consists of 4 carbon atoms
and 10 hydrogen atoms.
Structural formula
Structural formula of a compound represents the exact
arrangement of the different atomsof various elements present in a molecule of
a substance. In a structural formula, singlebond is represented by a single
line (-), a double bond by two lines (=) and a triple bond bythree lines ()
between the bonded atoms. Organic compounds may have same molecularformulae but
different structural formulae, e.g., structural formulae of butane C4H10
are:
Condensed Formula
The formula that indicates
the group of atoms joined together to each carbon atom in a straight chain or a
branched chain is called the condensed formula.
The formula which shows the sharing of electrons between various atoms in one molecule of the organic compound is called dot and cross formula or electronic formula.
Classification of Organic Compounds
All known organic
compounds have been broadly divided into two categories depending upon their
carbon skeleton. These are:
(i) Open chain or a cyclic
compounds.
(ii) Closed chain or
cyclic compounds.
(i) Open chain or a cyclic compounds
Open chain compounds are
those in which the end carbon atoms are not joined with each other, in this way
they form a long chain of carbon atoms. These chains may be either straight
or branched.For example,
(a) Straight
chain compounds are those in which carbon atoms link with each other through
a single, double or triple bond forming a straight chain such as;
(b) Branched
chain compounds are those in which there is a branch along a straight chain,
such as:
Open chain compounds are also called aliphatic compounds.
Closed chain or Cyclic compounds
Closed chain or cyclic
compounds are those in which the carbon atoms at the end of the chain are not
free. They are linked to form a ring. They are further divided into two
classes:
(a)
Homocyclic or carbocyclic compounds.
(b)
Heterocyclic compounds.
(a)Homocyclic or Carbocyclic compounds.
Homocyclic or carbocyclic
compounds contain rings which are made up of only one kindof atoms, i.e.,
carbon atoms. These are further divided into two classes:
• Aromatic compounds
• Alicyclic compounds
Aromatic compounds:
These organic compounds
contain at least one benzene ring in their molecule. A benzenering is made up
of six carbon atoms with three alternating double bonds. They are
calledaromatic because of aroma or smell they have. For example:
They are also called
benzenoid compounds.
Alicyclic or non-benzenoid compounds:
Carbocyclic compounds
which do not have benzene ring in their molecules are calledalicyclic or
non-benzenoid compounds. For example,
(b) Heterocyclic compounds
Cyclic compounds that
contain one or more atoms other than that of carbon atoms in theirrings are
called heterocyclic compounds.
Properties of Organic Compound
Organic compounds have the
following general characteristics:
(i) Origin:
Naturally occurring organic compounds are obtained from plants and animals.On
the other hand, inorganic compounds are obtained from minerals and rocks.
(ii) Composition: Carbon is an essential constituent of all organic compounds. They
aremade up of few elements such as carbon, hydrogen, nitrogen, oxygen, halogen,
Sulphur,etc. On the other hand, inorganic compounds are made up of almost all
the elements ofthe Periodic Table known so far.
(iii) Covalent linkage: Organic compounds contain covalent bonds, that may
be polar ornon-polar, while the inorganic compounds mostly contain ionic bonds.
(iv) Solubility:Organic compounds having non-polar linkages are generally soluble in
organic solvents likealcohol, ether, benzene, carbon disulphide etc. On the
other hand, the inorganic compoundswith ionic bonds are soluble in polar
solvents like water.
(v) Electrical conductivity:Due to the presence of covalent bonds, organic
compounds are poor conductors of electricity,whereas inorganic compounds being
ionic in nature, are good conductors of electricity in molten state or in
aqueous solution.
(vi) Melting and boiling points: Generally, organic compounds have low melting and
boiling points and are volatile in nature. Inorganic compounds, on the other
hand, have comparatively high melting and boiling points.
(vii) Stability: Since organic compounds have low melting and boiling points, they are
less stable than inorganic compounds.
(viii) Combustibility:
Organic compounds with high percentage of carbon are generally combustible.
On the other hand, inorganic compounds are mostly non-combustible.
(ix) Isomerism:
A main characteristic of organic compounds which differentiate them from
inorganic substances is their tendency to exhibit the phenomenon of isomerism.
Isomerism is rare in inorganic substance.
(x) Rate of reaction: Due to the presence of covalent linkages, the reactions of organic
compounds are molecular in nature. They are often slow and require specific
conditions such as temperature, pressure or catalyst.
International Union Nomenclature of Alpha
IUPAC
nomenclature is based on naming a molecule's longest chain of carbons
connected by single bonds, whether in a continuous chain or in a ring. All
deviations, either multiple bonds or atoms other than carbon and hydrogen, are
indicated by prefixes or suffixes according to a specific set of priorities.
α (Alpha) – the name given to the configuration of a cyclic sugar where the oxygen on the anomeric carbon is on the opposite face of the ring relative to the substituent on the other carbon flanking the ring oxygen. Contrasted with beta (β) which is where the two substituents are on the same faces of the ring.
Compounds (IUPAC)
In order to
name organic compounds, you must first memorize a few basic names. These names
are listed within the discussion of naming alkanes. In general, the base part
of the name reflects the number of carbons in what you have assigned
to be the parent chain. The suffix of the name reflects the
type(s) of functional group(s) present on (or within) the parent chain. Other
groups which are attached to the parent chain are called substituents.
Alkanes -
saturated hydrocarbons
Alkenes and
Alkynes - unsaturated hydrocarbons
·
Alcohols
·
Ethers
·
Aldehydes
·
Ketones
·
Carboxylic Acids
·
Amines
Functional Groups
An atom or
group of atoms or presence of double or triple bond which determines the
characteristic properties of an organic compound is known as the functional
group.
For example,
-OH group is the functional group of alcohols, which givescharacteristics
properties of alcohols. The characteristic properties of carboxylic acids are
dueto the presence of -COOH group in them. Therefore, functional group of
carboxylic acids is-COOH group.
In an organic compound, firstly, the
functional group is identified which gives us the appropriate suffix. Then the
longest carbon chain having the functional group is chosen in such a manner
that the functional group gets the lowest number in the chain. The priority
list of the functional group can be given as:
-COOH > -SO3H > -COOR (R=
alkyl group) > -COCl > -CONH2 > -CN > -HC=O
>>C=O > -OH > -NH2 > >C=C<> -CC-
Functional groups like -R, C6H5-, halogens (F, Cl, Br, I), -NO2, alkoxy (-OR) etc., are always used as prefix substituents.
1)Name the
compound given below.
·
In this compound,
the functional group present is –OH.
·
The longest chain
which contains the functional group has 8 carbon atoms. Hence, the saturated
hydrocarbon is octane.
·
The alcohol
functional group is present on the 3rd position, and a methyl group is on the 6th position.
·
Hence, the IUPAC
name will be 6- Methyloctan-3-ol.
2)Name the functional group given
below:
This compound has the
functional group as ketone (>C=O). Hence, the suffix will be one. And there
are two ketone groups. So, we will use di- before suffix as Dione. Continuing
in the same manner as above we get the name Hexane-2, 4-dione.
Names, Molecular, Condensed and Structural Formulae of the first ten Hydrocarbon
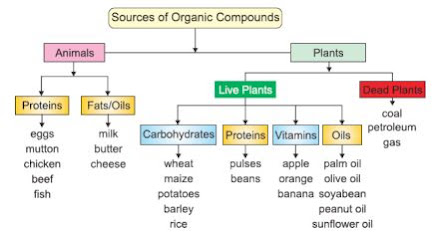
Sources Of Organic Compound
Organic compounds are prepared naturally by
animals and plants. Animals synthesize two main groups of organic compounds:
proteins and fats. Proteins are meat, mutton, chicken and eggs, etc. Fats are
present in milk, butter, etc. Plants synthesize; carbohydrates, proteins, fats,
vitamins, etc.
Moreover, dead plants buried under Earth’s crust are converted through biochemical processes to coal, petroleum and gas. These materials are the main sources of organic compounds. We can get thousands of organic compounds by the destructive distillation of coal and fractional distillation of petroleum. Details of each source are given in figure below:
Formation of Alkyl Radicals
Alkyl radicals are derivatives of alkanes. They
are formed by the removal of one of the hydrogen atoms of an alkane and are
represented by a letter ‘R’. Their name is written by replacing “ane” of alkane
with ‘yl’ represents first ten alkanes and their alkyl radicals.
Their general formula is Cn H2n+1
It is better to explain the type of radicals of
propane and butane. Propane has a straight chain structure. When terminal H is
removed, it is called n-propyl. When hydrogen from central carbon is removed,
it is called isopropyl, as explained below:
Similarly, different structures of butyl
radicals are explained:
A hydrocarbon
is an organic compound consisting of
hydrogen and carbon found in crude oil, natural gas, and coal. Hydrocarbons
are highly combustible and the main energy source of the world. Its uses
consist of gasoline, jet fuel, propane, kerosene, and diesel, to name just a
few.
Types of Hydrocarbons
There are two types of
hydrocarbons: aliphatic and aromatic. The three types of aliphatic hydrocarbons
are alkanes, alkenes, and alkynes. Aromatic hydrocarbons include benzene.
Overall, examples of hydrocarbons are methane, ethane, propane, and butane.
Alkanes
In organic chemistry, an
alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an
alkane consists of hydrogen and carbon atoms arranged in a tree structure in
which all the carbon–carbon bonds are single. Alkanes have the general chemical
formula CₙH₂ₙ₊₂.
Alkane as saturated hydrocarbons
Alkanes are a series of
compounds that contain carbon and hydrogen atoms with single covalent bonds. These
are known as saturated hydrocarbons. This group of compounds consists of carbon
and hydrogen atoms with single covalent bonds. Also comprises a homologous
series having a molecular formula of CnH2n+2.
Alkanes are the simplest
family of hydrocarbons. They contain only carbon and hydrogen. Each carbon atom
forms four bonds and each hydrogen atom forms one bond. Chemists use line-angle
formulas because they are easier and faster to draw than condensed structural
formulas. Structural formulas for alkanes can be written in yet another
condensed form.
The simple alkane methane contains one carbon atom and CH4 as its molecular formula. As this compound have just single covalent bonds only, therefore, its structural formula is
Alkenes and alkynes
Alkenes
have at least one carbon-carbon double bond. Alkynes have one or more
carbon-carbon triple bonds. Alkenes and alkynes are called as unsaturated hydrocarbons.
Alkenes
have at least one carbon-carbon double bond. Alkynes have one or more
carbon-carbon triple bonds. Alkenes and alkynes are called as unsaturated
hydrocarbons.Examples: Methane (CH₄), ethane (C₂H₆) is an example of
alkanes. Propane (C₃H₈), and ethene
are examples of alkene. Ethyne and
propyne are examples of alkyne.
Isomerism
In
chemistry, isomers are molecules or polyatomic ions with identical molecular
formula – that is, same number of atoms of each element – but distinct
arrangements of atoms in space. Isomerism is existence or possibility of
isomers. Isomers do not necessarily share similar chemical or physical
properties.
Types of isomerism:
1.Structural isomerism
2. Chain isomerism
1.Structural isomerism
2.
Chain isomerism
Functional groups containing carbon, hydrogen
and oxygen
Functional
Group Containing Carbon, Hydrogen and Halogens:
The organic compounds having functional group containing
carbon, hydrogen and halogens are called alkyl halides. Their functional group
is R-X. ‘X’ may be F, CI, Br or I
Macromolecule of human body
Proteins:In a cell, the most abundant macromolecules are proteins. Proteins are
long chains of amino acids essential for many biological functions, including structural
support, enzymatic activity, and signaling. They are involved in almost every
aspect of cellular function and are present in large quantities within cells.
Carbohydrates: Most people are familiar with carbohydrates, one type of macromolecule, especially when it comes to what we eat. To lose weight, some individuals adhere to “low-carb” diets. Athletes, in contrast, often “carb-load” before important competitions to ensure that they have enough energy to compete at a high level.
Carbohydrates are, in fact, an essential
part of our diet; grains, fruits, and vegetables are all natural sources of
carbohydrates. Carbohydrates provide energy to the body, particularly through
glucose, a simple sugar that is a component of starch and an ingredient
in many staple foods. Carbohydrates also have other important functions in
humans, animals, and plants.
Lipids: Lipids include a diverse group of compounds that are largely nonpolar in nature. This is because they are hydrocarbons that include mostly nonpolar carbon–carbon or carbon–hydrogen bonds. Non-polar molecules are hydrophobic (“water fearing”), or insoluble in water. Lipids perform many different functions in a cell. Cells store energy for long-term use in the form of fats.
Lipids also provide insulation from the environment
for plants and animals. For example, they help keep aquatic birds and mammals
dry when forming a protective layer over fur or feathers because of their
water-repellant hydrophobic nature. Lipids are also the building blocks of many
hormones and are an important constituent of all cellular membranes. Lipids
include fats, oils, waxes, phospholipids, and steroids.
Nucleic acids:Nucleic acids are the most important
macromolecules for the continuity of life. They carry the genetic blueprint of
a cell and carry instructions for the functioning of the cell.
DNA
and RNA
The two main types of nucleic acids
are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA
is the genetic material found in all living organisms, ranging from
single-celled bacteria to multicellular mammals. It is found in the nucleus of
eukaryotes and in the organelles, chloroplasts, and mitochondria. In
prokaryotes, the DNA is not enclosed in a membranous envelope.
In the other
type of nucleic acid, RNA, is mostly involved in protein synthesis. The DNA
molecules never leave the nucleus but instead use an intermediary to communicate
with the rest of the cell. This intermediary is the messenger RNA (mRNA).
Other types of RNA—like rRNA, tRNA, and microRNA—are involved in protein
synthesis and its regulation.
DNA and RNA
are made up of monomers known as nucleotides. The nucleotides combine with
each other to form a polynucleotide, DNA or RNA. Each nucleotide is made
up of three components: a nitrogenous base, a pentose (five-carbon) sugar, and
a phosphate group. Each nitrogenous base in a nucleotide is attached to a sugar
molecule, which is attached to one or more phosphate groups.
DNA Double-Helix Structure
DNA has a double-helix structure. The sugar and phosphate lie on the outside of the helix, forming the backbone of the DNA. The nitrogenous bases are stacked in the interior, like the steps of a staircase, in pairs; the pairs are bound to each other by hydrogen bonds.
Every base pair in the double helix is separated from the next base pair by 0.34 nm. The two strands of the helix run in opposite directions, meaning that the 5′ carbon end of one strand will face the 3′ carbon end of its matching strand. (This is referred to as antiparallel orientation and is important to DNA replication and in many nucleic acid interactions.)








Give your opinion if have any.